HARMONIC PERIODIC
SYSTEM AND GENETIC LAWS OF CHEMICAL ELEMENTS
Eng. Julio Antonio Gutiérrez Samanez
(ABSTRACT)
The Periodic Law of chemical elements that was first enounced by the russian chemist Dimitri Ivanovich Mendeleiev, in 1869, almost paralelly
with the german Lothar
Meyer, establishes that the properties of the chemical elements are periodic
functions of their atomic weigths. But this law is
only a theoretical enouncement not a law expressed in a mathematical function.
Based on the work of a great cusquenian
scientist, almost forgotten by official science, Dr. Oswaldo
Baca Mendoza (1908-1962), we have developed some ideas that try to meet that
mathematical expression, that, grouping and periodifying
the chemical elements, in function to their atomic numbers (Z) and other quantical parameters, show us what would be the formulas of
universal matter. We can synthetize the work as
follows:
Dr. Baca Mendoza proposed in his paper “Genetic laws of the chemical
elements: New Periodic System”,
Z = k + [1(n)] (1), for values k = 1 and n ³ 0,
being able to define the infinite natural series of
the formation of the nuclei of the chemical elements.
We know the main quantical number n represents
the floors or levels of energy of the atoms, at the same time each level of
energy has sublevels
an orbits defined by the quantical
numbers l, ml and ms. The sublevels are denoted by the letters s, p, d, f, g,
h, i
... etc, and contain the electrons ordered according the infinite series: 2, 6,
10, 14, 18, 22, 26, 30,... ¥
It is a recurrent series that results of the expression:
Number of electrons per sublevel = 2 (2n
– 1) to n ³ 1. (2)
One of the orbital orderings we will call “static” is as follows:
|
Levels of Energy |
K |
L |
M |
N |
O |
P |
Q |
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|
|
Sublevels |
1s |
2s 2p |
3s 3p 3d |
4s 4p 4d 4f |
5s
5p
5d 5f 5g |
6s
6p
6d 6f 6g 6h |
7s 7p 7d 7f 7g 7h 7I |
|
Nº of e- |
2 |
2
6 |
2
6 10 |
2
6 10 14 |
2 6
10 14 18 |
2 6
10 14 18 22 |
2 6
10 14 18 22 24 |
However the natural “dynamic” or harmonic ordering
know as “Aufbau” , that
shows the resulting “translappings” of the interactions
of energy in the sublevel, the increasing of the atomic number and the symmetry
effects, is what is seen in the series and following dispositions: (Levels of
energy, Notation of the sublevel, Quantical magnetical orbital number and Number of electron per
sublevel, respectively).
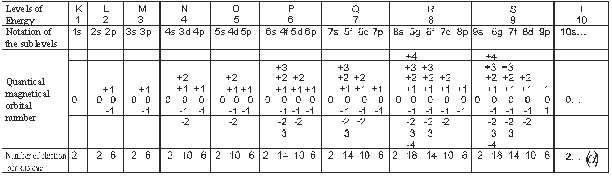
From this infinite series (a), we obtain
the Law of electronic configuration
Electronic configuration =2
(1,1,3,1,3,1,5,3,1,5,3,1,7,5,3,1,7,5,3,1,9,7,5,3,1,9,7,5,3,1.....) (3).
[where the number of electrons per sublevel
increases according to the expression (2)]
From the series (a), we can deduct two systems of harmonic periodification
A and B.
PERIODIFICATION SYSTEM A
(2); (2,6); (2,6); (2,10,6), (2,10,6); (2,14,10,6); (2,14,10,6);
(2,18,14,10,6); (2,18,14,10,6)....
2 8
8
18 18 32 32 50 50 ...... (b)
PERIODIFICATION SYSTEM
B
(2); (2); (6,2); (6,2); (10,6,2),
(10,6,2); (14,10,6,2); (14,10,6,2); (18,14,10,6,2); (18,14,10,6,2)....
2 2 8
8
18 18 32 32 50 50 ...... (g)
Let’s observe that in these series the electronic ordering also
corresponds, symmetrically, to the number of electrons existing in each period,
therefore, operating with the expression
(b) and (g) we obtain the periodic laws that Baca Mendoza called the genetic laws,
however, what I propose in this paper differs from what the researcher
proposed.
PERIODIC LAWS OR OF THE LIMITATION OF PERIODS
(PA) FOR
SYSTEM A
2 8 8 18 18 32 32 50 50 ...... (b)
2 2(22) 2(22) 2(32) 2(32) 2(42) 2(42) 2(52) 2(52)..... taking a common
factor:
PA = 2 (1, 22, 22, 32, 32, 42, 42, 52, 52..... ) (4)
(PB) FOR SYSTEM B
2 2 8 8 18 18 32 32 50
50
...... (g)
2 2 2(22)
2(22) 2(32) 2(32) 2(42) 2(42) 2(52) 2(52).....
PB = 2 (1, 1, 22, 22, 32, 32, 42, 42, 52, 52..... ) (5)
These Laws mathematically define the periodifications.
In the system A [series (b)], after the first term, 2 (Block of elements
from the first period), the periods are paired or binodic
(the expression binode was introduced by Dr. Baca):
2, 8 ,8, 18, 18, 32, 32, 50, 50, 72, 72, 98, 98,... and, in the system B,
all the periods are paired or binodic, therefore, they have an exact symmetry in their
increasing (2,2, 8, 8, 18,18, 32, 32,
50,50,...) ó (4, 16, 36, 64, 100, 144, 196....in binodes).
This binodic serie B,
reduces it self to the general parabolic expression or second grade function Y = 4m2. where Y is the growing up periodic function of m,
(m≥1), whish is number the couple of periods of binode.
Adding up the terms in the series we have Z = 4 ∑(mi)2
; for i=1 to n, and Z (atomic number)
With all
this we can design the periodic charts for both systems:
|
Sublevels
|
1s |
2s |
2p |
3s |
3p |
4s |
3d |
4p |
5s |
|
Atomic number (Z) |
1,2
|
3,4
|
5,6,7,8,9,10 |
11,12, |
13,14,15,16,17,18 |
19,20 |
21,22,23,24,25,26,27,28,29,30 |
31,32,33,34,35,36 |
37,38 |
|
Nº
elements |
2 |
2 |
6 |
2 |
6 |
2 |
10 |
6 |
2 |
|
4d |
5p |
6s |
4f |
5d |
|
39,40,41,42,43,44,45,46,47,48 |
49,50,51,52,53,54 |
55,56 |
57,58,59,60,61,62,63,64,65,66,67,68,69,70 |
71,72,73,74,75,76,77,78,79,80 |
|
10 |
6 |
2 |
14 |
10 |
|
6p |
7s |
5f |
6d |
7p |
|
81,82,83,84,85,86 |
87,88 |
89,90,91,92,93,94,95,96,97,98,99,100,101,102 |
103,104,105,106,107,108,109,110,111,112 |
113,114,115,116,117,118 |
|
6 |
2 |
14 |
10 |
6 |
|
|
|
|
|
|
We can distribute these ordered series by levels of energy in two
systems:
| SYSTEM A | SYSTEM B | |||
| Form A-1 | Form A-2 | Form B-1 | Form
B-2 |
|
| 1s | 1s | 1s | 1s |
|
| 2s 2p | 2s 2p | 2s | 2s |
|
| 3s 3p | 3s 3p | 2p 3s | 2p 3s |
|
| 4s 3d 4p | 4s 3d 4p | 3p 4s | 3p 4s |
|
| 5s 4d 5p | 5s 4d 5p | 3d 4p 5s | 3d 4p 5s |
|
| 6s 4f 5d 6p | 6s 4f 5d 6p | 4d 5p 6s | 4d 5p 6s | |
| 7s 5f 6d 7p | 7s 5f 6d 7p | 4f 5d 6p 7s | 4f 5d 6p 7s |
|
| 8s 5g 6f 7d 8p | 8s 5g 6f 7d 8p | 5f 6d 7p 8s | 5f 6d 7p 8s |
|
| 9s 6g 7f 8d 9p | 9s 6g 7f 8d 9p | 5g 6f 7d 8p 9s | 5g 6f 7d 8p 9s |
|
| 6g 7f 8d 9p 10s | 6g 7f 8d 9p10s |
The same distribution expressed in terms of maximum number of
differentiating electrons per level and sublevel, also corresponds to the
number of elements per levels of energy and blocks (s, p, d, f,..)
| SYSTEM A | SYSTEM B | |||
| Form A-1 | Form A-2 | Form B-1 | Form B-2 | |
| 2 | 2 | 2 | ||
| 2 6 | 2 6 | 2 | 2 |
|
| 2 6 | 2 6 | 2 6 | 2 6 |
|
| 2 10 6 | 2 10 6 | 2 6 | 2 6 |
|
| 2 10 6 | 2 10 6 | 2 10 6 | 2 10 6 |
|
| 2 14 10 6 | 2 14 10 6 | 2 10 6 | 2 10 6 |
|
| 2 14 10 6 | 2 14 10 6 | 2 14 10 6 | 2 14 10 6 |
|
| 2 18 14 10 6 | 2 18 14 10 6 | 2 14 10 6 | 2 14 10 6 |
|
| 2 18 14 10 6 | 2 18 14 10 6 | 2 18 14 10 6 | 2 18 14 10 6 |
|
| 2 18 14 10 6 | 2
18 14 10 6 |
The reader would observe that the increasing number of blocks of
elements is a function of the increasing number of electrons in the levels and
sublevels. (s, p, d, f, g)
LAWS
OF VERTICAL GROUPING OR OF GROUPS
This periodicity is functional and harmonic, with the Law of vertical grouping or synchronic of elements(Zg) that the Dr. Baca
called Law of groups that
is a result of adding up the terms in each one of the series (b)
and (g)
FOR SYSTEM A
Operating with the expression (b)
2
+ 8 +
8 + 18
+ 18 +
32 + 32
+ 50 +
50 ......
2
+ 2(22) + 2(22) + 2(32) + 2(32) +
2(42) + 2(42) + 2(52) + 2(52)..... Taking a common factor:
2 (1 + 22 + 22
+ 32 + 32 + 42 + 42 + 52 + 52
+..... )
For the resulting series to start
with the unit (Hydrogen, 1H),
we introduce zero in the sum and add an integer Z ³ 1 to the whole expression, to get:
ZgA = Z + 2 ( 0 + 1 + 22 + 22
+ 32 + 32 + 42 + 42 + 52 + 52
+..... ) (6)
For example for Z=1
ZgA = 1, 3, 11, 19,
37, 55, 87,....
That corresponds in the chart to the vertical group :
= 1H, 3Li, 11Na, 19K, 37Rb, 55Cs, 87Fr,.....
FOR SYSTEM B
Operating with the expression (g)
2 + 2 +
8 + 8 + 18
+ 18 +
32 + 32
+ 50 +
50 ......
2
+ 2 + 2(22)
+ 2(22) + 2(32) + 2(32) +
2(42) + 2(42) + 2(52) + 2(52)..... Taking a common factor:
2 (1 + 1 + 22 + 22
+ 32 + 32 + 42 + 42 + 52 + 52
+..... )
Like in the previous case, for the resulting
series to start with the unit, we introduce zero in the sum and add an integer Z ³ 1 to whole expression, which
results in:
ZgB = Z + 2 ( 0 + 1 + 1 + 22 + 22
+ 32 + 32 + 42 + 42 + 52 + 52
+........) (7)
To Z=1
ZgB= 1, 3, 5, 13,
21, 39, 57, 89, ....
= 1H, 3Li, 5B, 13Al, 21Sc, 39Y, 57La,
89Ac,.....
From the interaction of these laws
the harmonic systems or periodic charts are constructed, like it is shown
below, each one has its ow
variations. (Appendix 2.1 and 8.3)
Summarizing,
these laws show the universal validity of dialectics, by demonstrating that quantitative
changes in growing lead to qualitative changes or leaps from an inferior to a
superior level, in this case symetrical, paired and
exact. This increasing follows the interior dynamics of the atom, always from
inside towards outside, both in nuclear increasing as well as electronic
SPIRAL
ORDERING OF THE PERIODIC
SERIES OF ELEMENTS
FIRST LEVEL OR PERIOD (appendix 3.1, red colored curve)
![]() ;
; ![]()
A polar spiral is drawn from 0 a 2 π, which radium is a function of φ , and takes the value of 1(for hydrogen) for the angle π or de
180 degrees and the value of 2 for the angle 2 π, (360 degrees)
|
φ |
0 |
π/4 |
π/2 |
π |
3
π/2 |
2 π |
|
R1 |
0 |
0.25 |
0.5 |
1 |
1.5 |
2 |
|
Elements |
|
|
|
H |
|
He |
SECOND LEVEL (Appendix 3.1, orange
colored curve)
It is a spiral that starts in the radium 2,
like origin, and goes from
0 a 360 degrees or 2π
dividing in eight sections the circle in which it inscribes,
corresponding to eight elements from 3LI
al 10 Ne. According to the formula: R = 4/π φ; R2 = R+2
|
φ |
π/4 |
π/2 |
3π/4 |
π |
5π/4 |
3π/2 |
7π/4 |
2 π |
|
R |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
R2
= R+2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
|
Elements |
Li |
Be |
B |
C |
N |
O |
F |
Ne |
![]() ;
; ![]()
THIRD LEVEL (Appendix 3.1, yellow
colored curve)
Spiral that stars in the radium 10 at 0 degrees and goes on, dividing
the circle in witch it inscribes in eight sections , like in the previous case,
to the radium 18Ar. According to the formula: R = 4/π φ;
R3 = R+10
![]() ;
; ![]()
|
φ |
π/4 |
π/2 |
3π/4 |
π |
5π/4 |
3π/2 |
7π/4 |
2π |
|
R |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
R3=R+10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
|
Element |
Na |
Mg |
Al |
Si |
P |
S |
Cl |
Ar |
FOURTH LEVEL (Appendix 3.2, green
colored curve)
The spiral starts in the radium 18Ar and goes
to the radium 36Kr, in the angle φ= 2π, in a circle divided in 18 sections, according to the formula:
R = 9/π φ; R4 = R+18
![]() ;
; ![]()
|
φ |
π/9 |
2π/9 |
3π/9 |
4π/9 |
5π/9 |
6π/9 |
7π/9 |
8π/9 |
9π/9 |
10π/9 |
11π/9 |
12π/9 |
13π/9 |
14π/9 |
15π/9 |
16π/9 |
17π/9 |
18π/9 |
|
R |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
|
R4=R+18 |
19 |
20 |
21 |
22 |
23 |
24 |
25 |
26 |
27 |
28 |
29 |
30 |
31 |
32 |
33 |
34 |
35 |
36 |
|
Element |
K |
Ca |
Sc |
Ti |
V |
Cr |
Mn |
Fe |
Co |
Ni |
Cu |
Zn |
Ga
|
Ge |
As |
Se |
Br |
Kr |
FIFTH LEVEL (Appendix 3.2, Blue colored curve)
The spiral starts in the radium
36Kr. and goes on, again inscribed in a circle of 18 sections to the radium
54Xe, in the
angle 2π. According to the expression : R = 9/π φ; R5 = R+36
![]() ;
; ![]()
|
φ |
π/9 |
2π/9 |
3π/9 |
4π/9 |
5π/9 |
6π/9 |
7π/9 |
8π/9 |
9π/9 |
10π/9 |
11π/9 |
12π/9 |
13π/9 |
14π/9 |
15π/9 |
16π/9 |
17π/9 |
18π/9 |
|
R |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
|
R5=R+36 |
37 |
38 |
39 |
40 |
41 |
42 |
43 |
44 |
45 |
46 |
47 |
48 |
49 |
50 |
51 |
52 |
53 |
54 |
|
Element |
Rb |
Sr |
Y |
Zr |
Nb |
Mo |
Tc |
Ru |
Rh |
Pd |
Ag |
Cd |
In |
Sn |
Sb |
Te |
I |
Xe |
SIXTH LEVEL (Appendix 3..3, indigo colored curve)
The spiral starts en radium 54Xe, and goes
on now inscribed in a circle of 32
sections, to the radium 86Rn. In the angle 2π. According to the
expression:
R = 16/π φ;
R6 = R+54
![]() ;
; ![]()
|
φ |
π/16 |
2π/16 |
3π/16 |
4π/16 |
5π/16 |
6π/16 |
7π/16 |
8π/16 |
9π/16 |
10π/16 |
11π/16 |
12π/16 |
13π/16 |
14π/16 |
15π/16 |
16π/16 |
|
R |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
|
R6=R+54 |
55 |
56 |
57 |
58 |
59 |
60 |
61 |
62 |
63 |
64 |
65 |
66 |
67 |
68 |
69 |
70 |
|
Element |
Cs |
Ba |
La |
Ce |
Pr |
Nd |
Pm |
Sm |
Eu |
Gd |
Tb |
Dy |
Ho |
Er |
Tm |
Yb |
|
17π/16 |
18π/16 |
19π/16 |
20π/16 |
21π/16 |
22π/16 |
23π/16 |
24π/16 |
25π/16 |
26π/16 |
27π/16 |
28π/16 |
29π/16 |
30π/16 |
31π/16 |
32π/16 |
|
17 |
18 |
19 |
20 |
21 |
22 |
23 |
24 |
25 |
26 |
27 |
28 |
29 |
30 |
31 |
32 |
|
71 |
72 |
73 |
74 |
75 |
76 |
77 |
78 |
79 |
80 |
81 |
82 |
83 |
84 |
85 |
86 |
|
Lu |
Hf |
Ta |
W |
Re |
Os |
Ir |
Pt |
Au |
Hg |
Tl |
Pb |
Bi |
|
At |
Rn |
SEVENTH LEVEL (Appendix 3.3, violet
colored curve)
The spiral starts in radium 86Rn
, and goes inscribed in a circle also divides in 32 sections, to the
radio 118 Dsc (unknown rare gas) in the angle 2π. The formula is : R = 16/π φ;
R7 = R+86
![]() ;
; ![]()
|
φ |
π/16 |
2π/16 |
3π/16 |
4π/16 |
5π/16 |
6π/16 |
7π/16 |
8π/16 |
9π/16 |
10π/16 |
11π/16 |
12π/16 |
13π/16 |
14π/16 |
15π/16 |
16π/16 |
|
R |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
|
R7=R+86 |
87 |
88 |
89 |
90 |
91 |
92 |
93 |
94 |
95 |
96 |
97 |
98 |
99 |
100 |
101 |
102 |
|
Element |
Fr |
Ra |
Ac |
Th |
Pa |
U |
Np |
Pu |
Am |
Cm |
Bk |
Cf |
Es |
Fm |
Md |
No |
|
17π/16 |
18π/16 |
19π/16 |
20π/16 |
21π/16 |
22π/16 |
23π/16 |
24π/16 |
25π/16 |
26π/16 |
27π/16 |
28π/16 |
29π/16 |
30π/16 |
31π/16 |
32π/16 |
|
17 |
18 |
19 |
20 |
21 |
22 |
23 |
24 |
25 |
26 |
27 |
28 |
29 |
30 |
31 |
32 |
|
103 |
104 |
105 |
106 |
107 |
108 |
109 |
110 |
111 |
112 |
113 |
114 |
115 |
116 |
117 |
118 |
|
Lw |
Ku |
Ha |
Dcs* |
Dsc |
Dsc |
Dsc |
Dsc |
Dsc |
Dsc |
Dsc |
Dsc |
Dsc |
Dsc |
Dsc |
Pe** |
* Dsc : Unknown
** Hypothetical rare gas Peruvion:
honoring the country of the author
The levels or periods eighth and ninth will
have spirals inscribes in circles of 50 sections, (2 x 5x5)
![]() ;
; ![]()
The levels tenth and eleventh will
have spirals inscribed
in circles of 72 sections..
![]() ;
; ![]()
The periods twelfth and thirteenth will have
spiral inscribed in circles of 98 sections
![]() ;
; ![]()
Summarizing the general relation for system A,
this will be :
R = R1, R2, R3, R4, R5, R6, R7, ……
R = [ 1/π j];[4/π j+2];[4/π j+10];[ 9/π j+18];[9/π j +36];[16/π j + 54];[16/π j + 86]….
R=[1/π j];[22/π j +2];[22/π j +10];[32/π j+18];[32/π j+36];[42/π j+ 54];[42/π j+ 86]….
For system B the mathematical relation will be :
R =[1/π j]; [1/π j+2]; [22/π j +4];[22/π j +12];[32/π j+20];[32/π j+38];[42/π j+ 56];[42/π j+ 88]….
The following graphics are appendix for system
A.
This
is how we summarize our book: "Harmonic Periodic System and Genetic Laws
of Chemical Elements", to honor the memory of Dr. Oswaldo
Baca Mendoza, in whose creative work we were inspired, developing, improving
and overcoming it dialectically like the master would have liked, after half
century of conspiration of forgetfulness, silence and
incapability of assimilation by official science. In the book we expose with
the corresponding detail in each one of the topics, form more information, the
reader can access our
web page and to furthermore communicate your opinions and
comments to the e-mails
Some
"rough drafts" of the book have already been forwarded to colleagues
and prestigious scientist aiming for their authorized comments.
Eng. Julio Antonio Gutiérrez Samanez
Consultant in
Ceramics Technology specialized in
www.harras.be/hvar/kutiry ; kutiry@mixmail.com ; kutiry@hotmail.com
Calle Inca 357
Santiago Cuzco Perú
(El autor agradece la
traducción realizada por los ingenieros: Orestes Villafuerte
Romero y Juan Carlos Villafuerte Medina)